Frequently Asked Questions
A cancer medicines supplier primarily supports licensed institutions such as hospitals, oncology centers, pharmaceutical distributors, NGOs, and authorized procurement partners. Supply is conducted strictly through regulated, documentation-led channels for institutional use.
An anti cancer medicine exporter coordinates manufacturer-authorized sourcing, export documentation, and regulatory alignment to enable compliant cross-border oncology supply. This model supports distributors and institutions seeking structured international procurement rather than transactional sourcing.
An oncology medicine supplier supports institutional procurement by ensuring controlled sourcing, traceability, and regulatory consistency. Oncology medicines supply is managed to meet the operational requirements of licensed buyers and approved distribution networks.
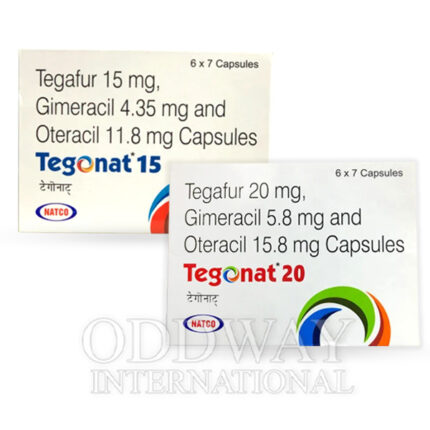
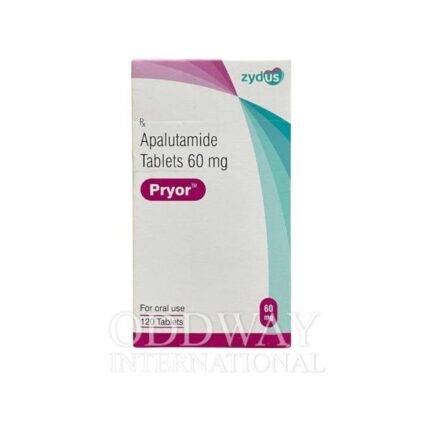
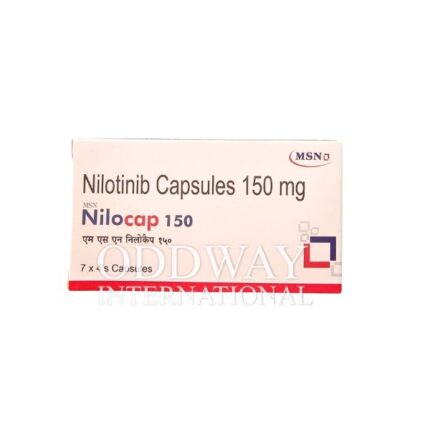
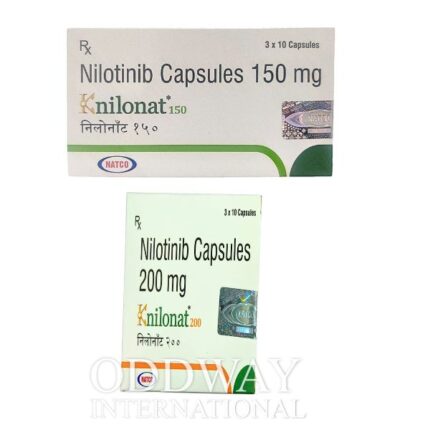
Oncology medicines from India are widely sourced due to established manufacturing capabilities, export-ready infrastructure, and experience in international pharmaceutical trade. Cancer medicine from India supports scalable and regulated supply for global oncology programs.
No. Oncology medicine exporters operate exclusively within B2B and institutional frameworks. Supply activities are limited to licensed entities and do not include medical advice, treatment recommendations, or direct patient services.
Showing 21–40 of 200 resultsSorted by latest